Cheng Zhang, Xin-Hu Hu, Ya-Hui Wang, Zhuo Zheng, Jie Xu, and Xiang-Ping Hu
J. Am. Chem. Soc.
DOI: 10.1021/ja303129s
Bridged bicyclic structures are common motifs in many naturally occurring and bioactive compounds. As such, these systems are attractive targets for synthetic chemists. However, their highly shape-defined structure poses an interesting synthetic challenge. Common approaches in preparation of bridged systems include regiospecific Heck couplings and carbenoid addition-Claisen rearrangement cascades.
These approaches, although reliable, are characterized by long syntheses of the cyclization substrates.
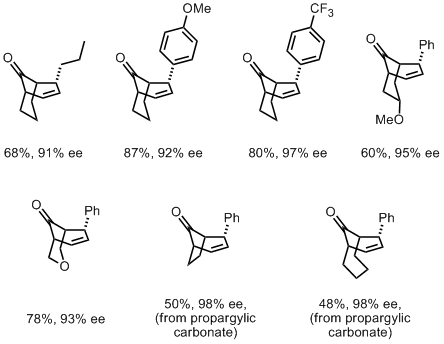
A group from the Chinese Academy of Sciences in Beijing has recently reported a diastereo- and enantio-selective Cu(II)-catalysed formal [3+3] cycloaddition for the preparation of bicyclo(n.3.1) structures from simple propargyl esters and cyclic enamines. Treatment of the propargyl ester with Cu(OAc)2 in the presence of a chiral tridentate ligand yield a very reactive allenylidene intermediate, which undergoes cycloaddition in the presence of an enamine nucleophile.
The reaction is shown to work best with six membered cyclic enamines, which can also contain heteroatoms. However, decent yields were also obtained with five and seven membered nucleophiles.
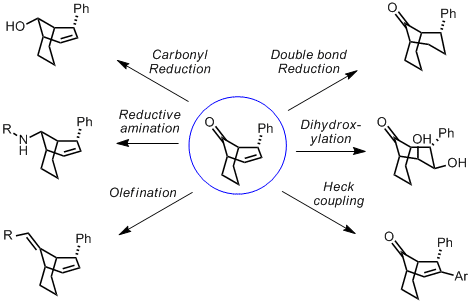
The bicyclo(n.3.1) products can be functionalised stereoselectively either through C-C double bond chemistry or carbonyl chemistry and serve as building blocks for the synthesis of more complicated systems.



I enjoyed reading your blog, but it’s been quite a while since your last post. Are you taking the summer off?
Yep, there’s been a bit of a break. However, George K has written up a couple of posts that I need to put up, and I have a reading list of ~20 browser tabs, so we should be back up to speed pretty soon.
Having said that, we have been keeping our eye out for anyone who might be interested in joining us. There’s simply too much good chemistry for a few guys to keep up with.